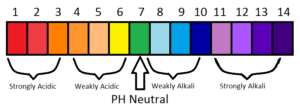
Acids are defined as compounds that donate a hydrogen ion (H+) to another compound (called a base). Acids have a pH less than 7.0 on pH scale.
Bases are the chemicals that, when dissolved in water, give a solution with a hydrogen ion activity lower than that of pure water, i.e. a pH higher than 7.0 at standard conditions.
A reaction between an acid and a base is called neutralization (उदासीनीकरण) and this neutralization results in production of water and a salt (लवण).
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
(Base) (Acid) (Salt) (Water)
Acids in general are H+ donors and Bases are H+ acceptors.
|
Acids |
Bases |
|
1. pH Less than 7.0 |
1. pH greater than 7 and go upto 14 |
|
2. Remain colorless with Phenolphthalein |
2. Show pink color with Phenolphthalein |
|
3. Change Litmus paper red |
3. Change Litmus paper blue |
|
4. Sour in taste |
4. Bitter in taste |
|
5. Free hydrogen ions (H+) when mixed with water |
5. Free hydroxide ions (OH-) when mixed with water |
| 6. Strong acids have a corrosive effect on metals | 6. Strong bases have a caustic effect on organic matter |
| 7. Acids can be classified as Mineral acids, Sulfonic acids, Carboxylic acids, Vinylogous carboxylic acids and Nucleic acids. | 7. Bases are of 2 types – a base and an alkali (a soluble base). |
| 8. Examples: Hydrochloric acid (HCl), Sulfuric acid (H2SO4), Nitric Acid (HNO3) Etc. | 8. Potassium Hydroxide (KOH), Sodium Hydroxide (NaOH), Magnesium Hydroxide (Mg (OH)2) Etc. |
| 9. Used as additives in food and beverages industry, to remove rust from metals, as an electrolyte in batteries, for mineral processing, to produce fertilizers and gasoline etc. | 9. to manufacture soap, paper, synthetic fiber, bleaching powder, remove grease stains from clothes etc. |


